
Convert grams Na2CO3 to moles or moles Na2CO3 to grams Molecular weight calculation: 22.989772 + 12.0107 + 15.99943 Percent composition by element Similar chemical formulas Note that all formulas are case-sensitive. Molar mass of NaCl atomic mass of Na (22.99 amu) + the atomic mass of Cl (35.45. Na2CO3 molecular weight Molar mass of Na2CO3 105.98844 g/mol This compound is also known as Sodium Carbonate. Thus, the molar mass of bilirubin can be expressed as 584.73 g/mol, which is read as “five hundred eighty four point seventy three grams per mole. So, one mole of water (6.022 x 10 23 molecules) has a mass of 18.02 g. The division sign (/) implies “per,” and “1” is implied in the denominator. Molar mass of Na (OH)2 is 57.00445 0.00074 g/mol Get control of 2022 Track your food intake, exercise, sleep and meditation for free. Sodium Selenite Composition and Molar Mass Chemical formula Molar mass of Na 2 SeO 3, Sodium Selenite is 172.93774 g/mol 22.989772+78.96+15.99943 Mass percentage of the elements in the composition Notes on using the Molar Mass Calculator Chemical formulas are case-sensitive. For example, the molar mass of Ba(OH) 2 requires the sum of 1 mass of Ba, 2 masses of O, and 2 masses of H: The molar mass of Ba(OH)2 requires the sum of 1 mass of Ba, 2 masses of O, and 2 masses of H: 1 Ba molar mass:īecause molar mass is defined as the mass for 1 mol of a substance, we can refer to molar mass as grams per mole (g/mol). In formulas with polyatomic ions in parentheses, the subscript outside the parentheses is applied to every atom inside the parentheses.

Molar mass of Na (cation) is 22.989769280 ± 0.000000020 g/mol. Molar mass of NaSO4 is 119.0524 g/mol Get control of 2022 Track your food intake, exercise, sleep and meditation for free.
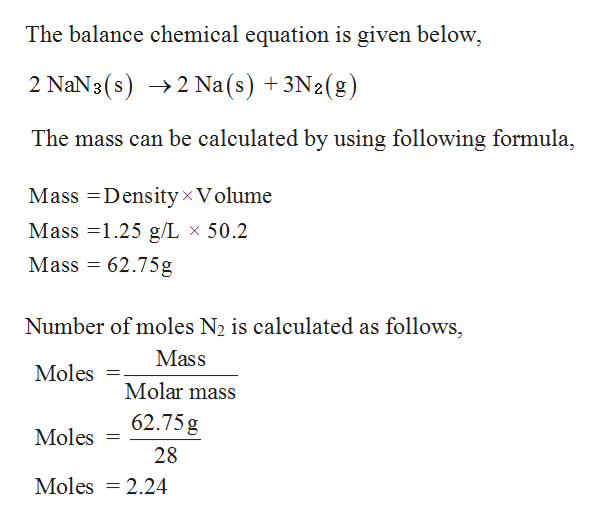
barium sulfate (BaSO 4), used to take X rays of the gastrointestional tract Molar Mass, Molecular Weight and Elemental Composition Calculator.Remember In some books, you may see the unit of molar mass as grams/mole or g/mole. I hope you have understood the short and simple calculation for finding the molar mass of NaCl.
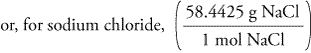
For example, the molar mass of NaCl can be calculated for finding the atomic mass of sodium (22.99 g/mol) and the atomic mass of chlorine (35.45 g/mol) and combining them. What is the mass of 1 mol of each substance? So, Molar mass of NaCl Molar mass of 1 Sodium (Na) atom + Molar mass of 1 Chlorine (Cl) atom. To calculate the molar mass of a compound with multiple atoms, sum all the atomic mass of the constituent atoms. \): Moles to Mass Conversion with Compounds Molar mass of Na is 22.989769280 0.000000020 g/mol Compound name is sodium Get control of 2022 Track your food intake, exercise, sleep and meditation for free.


 0 kommentar(er)
0 kommentar(er)
